Jackson Scheppers
Scientist, KBI Biopharma, Louisville, CO

Damian Guerra
Sr. Scientist and Group Leader, KBI Biopharma, Louisville, CO
“Careful, consistent sample preparation begets reliable peptide mapping data,”
Monoclonal antibodies (mAbs) comprised 26 % of new drugs approved by the US Food and Drugs Administration (FDA) in 2024 [1], a new record for this modality. Despite exhibiting high overall sequence conservation, each therapeutic mAb requires an integrated control strategy to achieve safety and efficacy. Unique complementarity-determining region (CDR) sequences produce intrinsic differences among mAbs, which are augmented by iterative optimization of cell lines, media, and purification procedures. Process and product development strategies can influence post-translational modifications (PTMs)—e.g., glycosylation type and pattern, canonical and non-canonical disulfide bonds, aspartate isomerization, and oxidation of methionine and tryptophan residues—some of which may impinge on critical quality attributes (CQAs).
A key analytical method to assess primary structure CQAs is peptide mapping, by which proteins are proteolyzed into peptides that are separated and detected by liquid chromatography-mass spectrometry (LC-MS). Along with sequence confirmation and disulfide assessment, peptide maps can quantify PTM abundance by expressing abundance ratios of modified to native peptides.
KBI Biopharma has provided trusted analytical services for nearly three decades, supporting over 1400 clients ranging from Top 20 Pharma companies to VC-funded biotech startups. , reflecting the company’s reputation for handling the industry’s most challenging analytical requirements. While monoclonal antibodies represent routine analytical work for KBI’s experienced teams, the company’s portfolio spans far more complex molecular modalities including antibody-drug conjugates, bispecific antibodies, gene therapies, cell therapies, and novel protein therapeutics. KBI provides comprehensive analytical characterization from discovery through commercial manufacturing, with primary structure analyses representing just one component of their integrated analytical capabilities across multiple sites in Colorado, North Carolina, and Geneva. Exemplifying KBI’s expertise is Jackson Scheppers, a scientist at KBI-Louisville, Colorado with an extensive background in protein and peptide mass spectrometry.
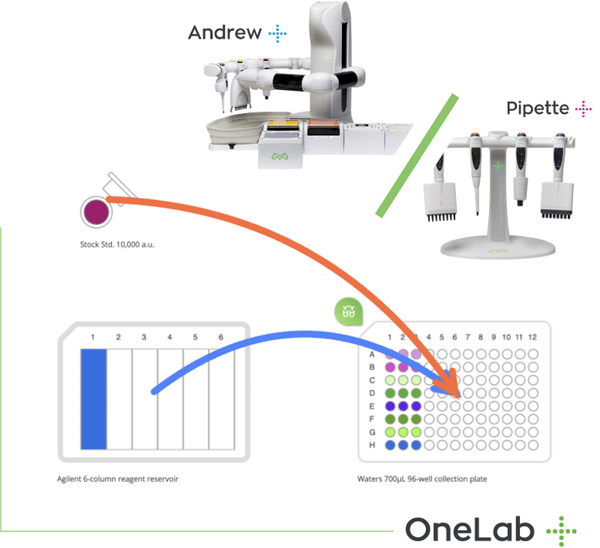
“We explored laboratory automation strategies to curtail method-induced variability and artefacts,” said Dr. Damian Guerra, senior scientist group leader at KBI-Louisville. “Among automation platforms, we chose the Andrew+ Robot for its physical versatility and the ease of procedure composition using the OneLabTM Software. With little to no experience in scripting languages like Python, we could easily write protocols for Andrew+ Robot.”
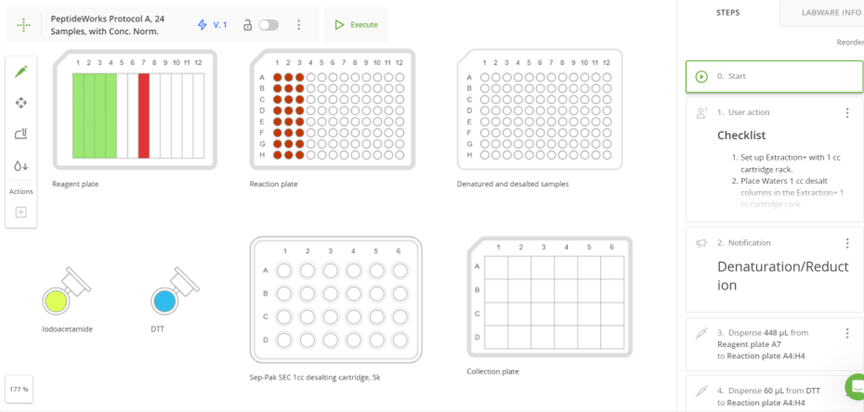
A screenshot of protocol developed in OneLab Software that Jackson Scheppers uses to perform proteolysis of mAbs prior to LC-MS analysis.
“OneLab Software is a cloud-based software platform built for collaboration. This is particularly useful for KBI, as we have sites in Colorado, North Carolina, and Geneva. OneLab Software will enable us to develop and share procedures for Andrew+ Robots at different sites and thus increase method precision,” stated Mr. Scheppers.
Over the past year Mr. Scheppers has explored multiple strategies to adapt the Andrew+ Robot to routine liquid handling. One investigation found that mAb samples prepared with Andrew+ Robot produced less artefactual methionine oxidation than those prepared manually by a human analyst. These findings were detailed in a poster presented at the Society for Laboratory Automation & Screening (SLAS) Conference in San Diego in Jan. 2025 [2].
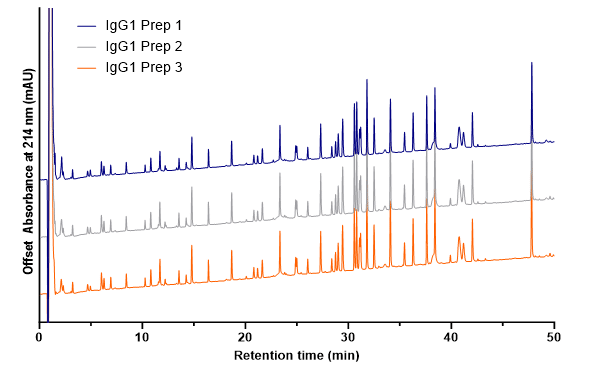
Using the Andrew+ Robot, powered by OneLab Software, Jackson Scheppers was able to obtain excellent preparation-to-preparation reproducibility when analyzing mAb digests using LC-UV and LC-MS.
“The Andrew+ Robot and Jackson can both do an excellent job of preparing mAb samples for peptide mapping studies. Andrew+ doesn’t replace human analysts, but it saves them time to focus on higher order endeavors such as experimental design and data analysis,” said Dr. Guerra. “Thankfully, Jackson knows where to turn when he needs an extra pair of hands.”
Andrew+ and OneLab are trademarks of Waters Technologies Corporation.
References:
- de la Torre, Beatriz G., and Fernando Albericio. “The Pharmaceutical Industry in 2024: An Analysis of the FDA Drug Approvals from the Perspective of Molecules.” Molecules3 (2025): 482.
- 2. Scheppers, J. Assessing the Repeatability and Quality of an Automated Peptide Mapping Workflow for use with Monoclonal Antibodies, Poster # 1125-B, SLAS Conference, Jan.27, 2025.